One-step Purification For Live Attenuated Rabies Virus Vaccine
Rabies is a preventable viral disease most often transmitted through the bite of a rabid animal. The rabies virus infects the central nervous system of mammals, ultimately causing disease in the brain and death (CDC). In up to 99% of cases, domestic dogs are responsible for rabies virus transmission to humans. Yet, rabies can affect both domestic and wild animals. It is spread to people through bites or scratches, usually via saliva. Without a rabies vaccine, the fatality rate is nearly 100% fatality rate when humans and pets are infected. (World Health Organisation). Rabies vaccines are the most effective method to protect humans and pets when they are exposed.
Compared with traditional rabies vaccine purification (membrane separation and size exclusion), Biovanix Prosep AF media can purify the live attenuated rabies virus in just one step, with better virus protein recovery and purity.
Rabies Vaccine Purification Tests
Result
- Recovery > 80%
- Purity > 90%
- Removal rate of impurities > 99%
Test Condition:
Column: Prosep 1ml pre-packed column
Mobile phase A: 10mM PB pH7.4
Mobile phase B: 10mM PB pH7.4 2M NaCl
Flow Rate: 0.5ml/min
Testing: UV 280nm
Sample: Clarified virus medium
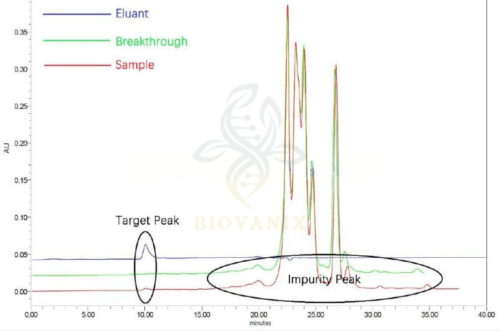
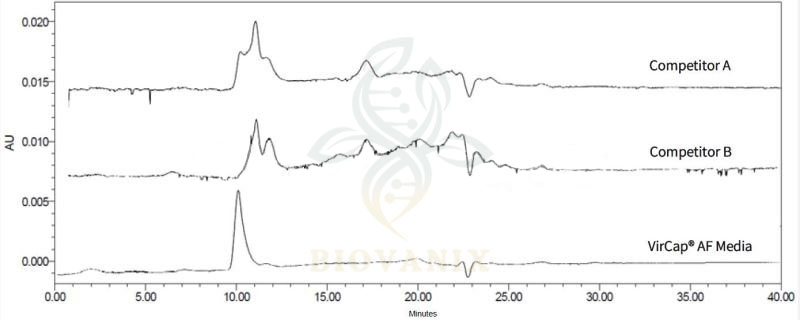
Using HPSEC analysis for the tests of sample solution, percolation fluid and collection fluid, and no peak of target in breakthough at RT=10min. Prosep AF media elutes virus particles by changing the ion strength in mobile phase. Both the purity and concentration of the virus improves significantly.
Parallel tests of HPSEC analysis for target peak collection is also done among competitors.
CIP and Clearance
Frequency of Clean-in-Place (CIP) depends on properties and conditions of solvent.
For the capture step, we suppose at least 30 minutes of 1 M NaOH countercurrent CIP process after each running.
To reduce microbial contamination in the prepacked column, clean with 0.5 to 1.0 M NaOH, 1 h contact time is recommended.